
Photograph of a radioactive Puffy Surgeon Fish in the Bikini Lagoon, following the the Baker shot of Operation Crossroads, Bikini Atoll, July 25, 1946. The speciment was placed on photographic film overnight and produced its own x-ray.
Radiation, radioactivity, irradiation — these terms, whether encountered in the fiction we consume or the news we receive, should have some level of familiarity. Their meaning, however, are far more complex than they appear, thereby adding to the difficulty of understanding the atomic circuit and its effects on our lives.
Radiation, first, simply designates the energy that moves through space. There are different types of radiation that take different forms — either as wave or as particle.
- Non-ionizing radiation (such as light, radio waves, microwaves) is a type of energy that is low, and therefore not strong enough to impact the structure of atoms. It can, however, make molecule vibrate and produce heat.
- Ionizing radiation (such as gamma-rays, X-rays, or alpha-rays) is a type of energy strong enough to impact matter at the subatomic levels, detaching the electrons from neutrons, for example. In concentrated and large doses, ionizing radiation is dangerous as it alters the fabric of life by destabilizing atoms.
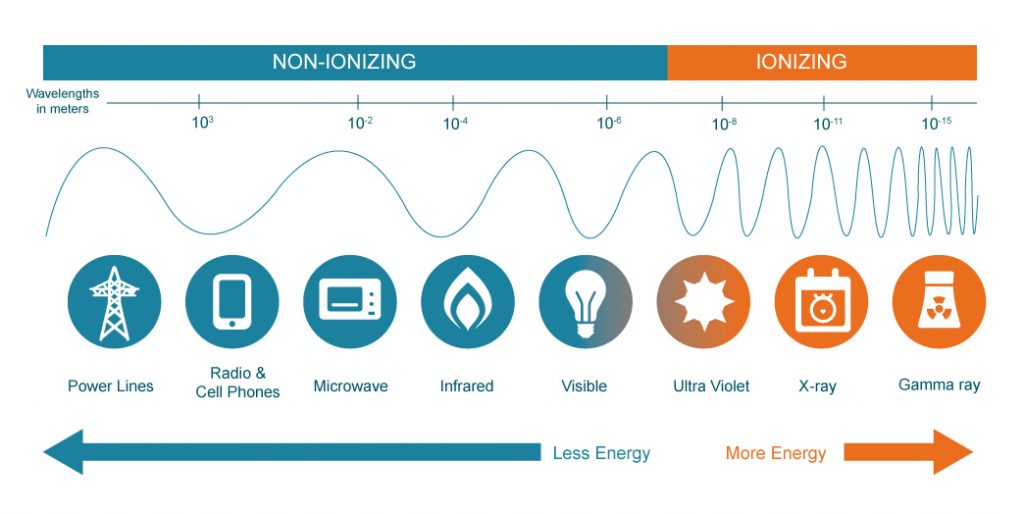
When atoms become unstable under the effects of ionizing energy, they seek to revert to a more stable form, releasing energy in the process. The energy released is quantifiable through radioactivity.
Irradiation, finally, describes the effects of radioactivity on a given substance. As energy passes through a substance (for instance, gamma ray passing through our body), a certain quantity is absorbed by the tissues. This absorption results in a level of irradiation that can range from low to high.
Measuring radioactivity:
Radioactivity is most often measured in Becquerels (though it is sometimes expressed in Curies). In essence, Becquerels describe the number of atomic nuclei that decay every second through a simple expression: 1 Bq = 1 nucleus decayed per second.
The unit, however, is misleadingly small. For example:
– 1 kg of granite = 1,000 Bq (a level equivalent to 1 kg of coffee)
– 1 kg of uranium = 25,000,000 Bq (or 25 MBq)
– 1 gram of Plutonium-238 = 640,100,000,000 Bq (or 640.1 GBq)
Measuring irradiation:
When radiation passes through bodies, a certain quantity is absorbed. The effects of that absorption have different expressions depending on the body measured. To measure the effects of radiation on humans, Sieverts is most commonly used. Unlike Becquerels, however, Sieverts are large units, and most effects are measured in millisieverts, or mSv.
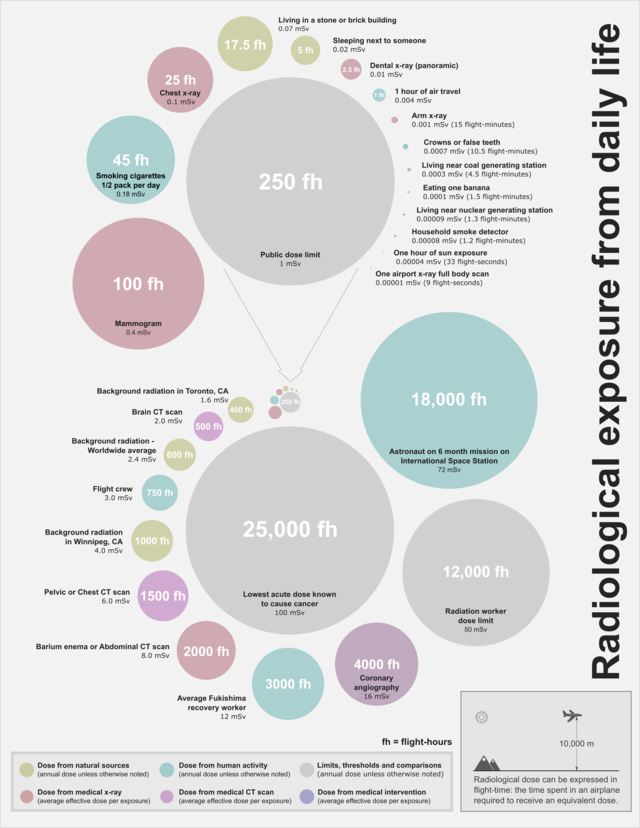
Radioactivity and irradiation, then, do not have an easy conversion ratio. The average human body is exposed to 2.6 mSv per year (this number varies from country to country), and it is not recommended for specialized workers to be exposed to more than 50 mSv per year. It is large doses of radiation delivered at once (and even on prolonged periods of time) that cause health issues. A concentrated exposure to 100 mSv (or 1 Sv) causes acute radiation sickness while a dose of 10 Sv is fatal.

Drone image of the Fukushima exclusion zone. Credits: Arkadiusz Podniesiński.